 Greenhouse gases are gases found in the atmosphere that are capable of absorbing infrared radiation emitted from the Earth’s surface and then emitting radiation back towards Earth, causing warming at the surface and lower atmosphere – the greenhouse effect.
Greenhouse gases are gases found in the atmosphere that are capable of absorbing infrared radiation emitted from the Earth’s surface and then emitting radiation back towards Earth, causing warming at the surface and lower atmosphere – the greenhouse effect.
Greenhouse gases, once present in the atmosphere, do not degrade quickly. Most greenhouse gases persist for many years, and thus contribute to warming for an extending period of time.
Key GHGs
Greenhouse gases differ in their atmospheric concentration, their residency time in the atmosphere, and their efficiency at absorbing and emitting longwave radiation.
The key GHG are:
Water vapour
Water vapour is the most abundant greenhouse gas in the atmosphere, and is a very effective absorber of radiation. As a result, water vapour plays an important contributing role in global warming.
Water vapour increases in the atmosphere as atmospheric temperatures increase. This is because warmer air is able to hold more moisture.
Unlike other greenhouse gases, the additional water vapour in the atmosphere was not put there directly by humans. The increase in water vapour occurs because the climate is warming, and the increase then contributes to further warming. This process is referred to as a positive feedback.
Also unlike other greenhouse gases, water vapour does not persist in the atmosphere. Excess water vapour soon falls out of the atmosphere as precipitation. Water vapour’s lifetime in the atmosphere can be measured in days, not decades or centuries as with other greenhouse gases. The water vapour that is in the atmosphere today will not continue to contribute to warming well into the future.
Carbon Dioxide (CO2)
Carbon dioxide levels in the atmosphere have increased dramatically in recent times – from approximately 280 ppm (parts per million) in pre-industrial times to approximately 400 ppm today. This represents an increase of more than 40%.
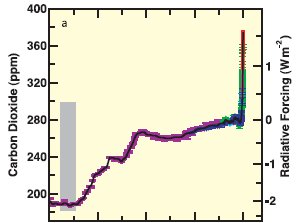
Figure 1: Carbon Dioxide levels (in ppm) and associated radiative forcing from 2005 back 20,000 years. (IPCC3)
The increase in carbon dioxide in the atmosphere shown in Figure 1 (1) is due primarily to the burning of fossil fuels and land use changes.
- Fossil fuels – Since the industrial revolution, humans have been relying increasingly on fossil fuels for transportation needs, industrial production, and for heat.
- Land use changes – Land use changes such as clearing land to make way for agriculture or for urban development, has also contributed to an increase in carbon dioxide levels. Clearing trees and replacing the land with cities or with agricultural land removes a valuable carbon sink. Trees are responsible for the uptake of large amounts of carbon dioxide. Once they are cleared, we have lost a valuable tool for offsetting some of the carbon dioxide that we are releasing into the environment.
Methane (CH4)
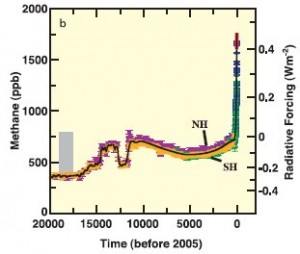
Figure 2: Methane levels (in ppb) and associated radiative forcing from 2005 back 20,000 years. (IPCC3)
Methane levels in the atmosphere shown in Figure 2 (1) have increased from approximately 715 ppb (parts per billion) in pre-industrial times, to approximately 1803 ppb in 2011.(2) This represents a staggering increase of over 150%.
The increase in methane in the atmosphere is caused primarily by human activities, such as livestock operations, rice agriculture, fossil fuels, biomass burning and decomposing garbage at landfills.
Nitrous Oxide (N2O)
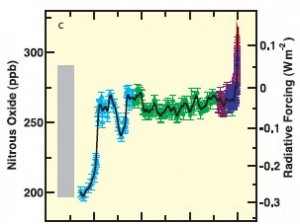
Figure 3: Nitrous oxide levels (in ppb) and associated radiative forcing from 2005 back 20,000 years. (IPCC3)
Nitrous oxide levels in the atmosphere shown in Figure 3 (1) have increased from approximately 270 ppb in pre-industrial times to approximately 319 ppb in 2005. This represents an increase of about 18%.
The increase of nitrous oxide into the atmosphere can be traced to the burning of fossil fuels, as well as the use of chemical fertilizers.
Halocarbons
Halocarbons are synthetic compounds that are created by humans. These chemicals have many applications including fire retardants and for air conditioners.
Halocarbons include compounds like:
- CFCs (chlorofluorocarbons)
- HCFCs (hydrochlorofluorocarbons)
- HFCs (hydrofluorocarbons)
Halocarbons are highly effective greenhouse gases as they absorb more radiation than other greenhouse gases. (Sometimes thousands of times more than CO2)
These compounds also tend to persist in the environment for longer than other greenhouse gases as it takes longer for them to break down.
CFCs are known ozone depleting substances. As a result, the international community has taken steps to ban the production and release of CFCs. The Montreal Protocol has yielded positive results in reducing the amount of CFCs released into the environment.
However, this ban resulted in a greater dependence on HCFCs and HFCs, which are powerful greenhouse gases. Processes are in place to phase out some of these products as well, but the fact that they persist in the atmosphere means they will continue to contribute to warming for centuries to come.
Click here for Manitoba greenhouse gas emission data.




